
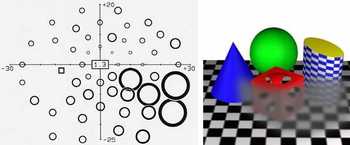
 a unilateral, relative arcuate scotoma,
onto a picture of the environment. A blurring-out of fine detail, a reduction of brightness, a dilution of contrast, and a decrease of saturation inside the defect borders conceivably could add some realism. Again, patients very rarely describe their perceptions in similar ways. The field defect depicted to the right by high-pass resolution perimetry was not visible to the patient, a highly educated subject, not even at a later, still more advanced stage. The differences between the perimetric and the subjective points of view are at least in part attributable to differences between vision at threshold (in the perimeter) and vision above threshold (in real life).
a unilateral, relative arcuate scotoma,
onto a picture of the environment. A blurring-out of fine detail, a reduction of brightness, a dilution of contrast, and a decrease of saturation inside the defect borders conceivably could add some realism. Again, patients very rarely describe their perceptions in similar ways. The field defect depicted to the right by high-pass resolution perimetry was not visible to the patient, a highly educated subject, not even at a later, still more advanced stage. The differences between the perimetric and the subjective points of view are at least in part attributable to differences between vision at threshold (in the perimeter) and vision above threshold (in real life).
 the memory of what has just been seen on looking around are likely to work against self-detection. Perceptual completion or "filling-in" may play an important role and so may the phenomenon of selective attention. For example, a patient with a complete right homonymous hemianopia who is looking at a familiar face may well fail to explicitly note that the right half actually is missing. For some sobering demonstrations of what tricks selective attention may play, pay a visit to the
Visual Cognition Lab
at the University of Illinois. This is the home of a famous
ball game video,
where the task is to count the number of passes made during the brief game. Afterwards, ask yourself if you saw something unexpected appear about halfway through. If not, play again. Highly recommended.
the memory of what has just been seen on looking around are likely to work against self-detection. Perceptual completion or "filling-in" may play an important role and so may the phenomenon of selective attention. For example, a patient with a complete right homonymous hemianopia who is looking at a familiar face may well fail to explicitly note that the right half actually is missing. For some sobering demonstrations of what tricks selective attention may play, pay a visit to the
Visual Cognition Lab
at the University of Illinois. This is the home of a famous
ball game video,
where the task is to count the number of passes made during the brief game. Afterwards, ask yourself if you saw something unexpected appear about halfway through. If not, play again. Highly recommended.
 Although many patients are unaware of their visual deficits, others are indeed aware but may report on phenomena that are poorly reflected by clinical test results.
For example, normal binocular summation may be replaced by binocular inhibition, i e, vision may be worse with both eyes together than with either eye alone. Similarly, it is a greater handicap to loose vision in the dominant eye than in the non-dominant one. Further, there may occur both static and dynamic disorders of stereo vision. These may be restricted to certain portions of visual space. These and many other abnormal percepts are often vaguely described and often in words that easily come to mind, e g, blur and double vision.
Although many patients are unaware of their visual deficits, others are indeed aware but may report on phenomena that are poorly reflected by clinical test results.
For example, normal binocular summation may be replaced by binocular inhibition, i e, vision may be worse with both eyes together than with either eye alone. Similarly, it is a greater handicap to loose vision in the dominant eye than in the non-dominant one. Further, there may occur both static and dynamic disorders of stereo vision. These may be restricted to certain portions of visual space. These and many other abnormal percepts are often vaguely described and often in words that easily come to mind, e g, blur and double vision.
There can be little doubt that blurring of central vision most often is caused by optical faults like ametropias and media opacities. Optical blur can easily be mimicked using optical over-correction and will be left aside here. Mimicking neural blur is a bit more complicated. It is presumably most often attributable to a loss of image detail by under-sampling, from a partial loss (or dysfunction) of neural channels. Neural channels may sustain damage anywhere along their course from the retinal receptors to the visual cortex. There is little to indicate that the location of damage influences the character of undersampling, so the following simulation may have a general application. The severity of damage can be selected in four steps by clicking the mouse in the text below: right-click to increase damage and left-click to decrease damage. Move the mouse pointer over the text to move the affected area. Note that it takes a keen observer to see small degrees of deficits, suggesting that subjective recognition generally will be late.
A strictly quantitative simulation of neural channel loss is available elsewhere on this site [A].
Dysmetropia means seeing that objects have unaccustomed dimensions. Micropsia is more common than macropsia. The two may be mixed, often in a spatially irregular fashion, to produce metamorphopsia. The below paintings were made by a patient suffering from a unilateral macular condition. The left painting shows a scene as seen by the normal eye. The right painting depicts metamorphopsia in the affected eye.

Dysmetropia is usually localized to the central-most visual field. It may be exceedingly annoying, not the least because the patch of distorted vision follows the gaze wherever it goes. Click the left mouse button over the display below to see see what 80% micropsia may look like. Click the right mouse button to set the scene for 120% macropsia instead. Move the mouse pointer to move the dysmetropic area over the text.
Dysmetropia is usually attributable to dislocation of photoreceptors. An increased separation causes the retinal image to involve a smaller than normal number of photoreceptors, setting the scene for micropsia. The opposite reasoning applies for macropsia.
A simple subjective comparison of apparent sizes between the eyes may be helpful to elucidate obscure visual problems. One way is to use alternating occlusion while looking at an acuity chart. It is best to direct the subject to judge an optotype size that is far above the resolution threshold, say, twice as large, to facilitate concentration on the comparison of size.
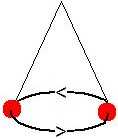 The classical Pulfrich phenomen refers to a peculiar distortion of binocular vision that might be labelled dynamic stereo dysmetropia. The phenomenon is most commonly elicited by means of a pendulum that is made to swing in the subject's frontal plane. When a light-attenuating filter is put in front of one eye, the pendulum appears to swing in and out of the plane, following a more or less ellipsoidal trajectory. The Pulfrich phenomenon may also arise spontaneously, for example, in the presence of optic neuropathy, and may then cause multiple problems in daily life, especially when judging paths of moving objects. A truly monocular counterpart called the Lazy Shadow has been discovered recently. See the Pulfrich page for more information [B].
The classical Pulfrich phenomen refers to a peculiar distortion of binocular vision that might be labelled dynamic stereo dysmetropia. The phenomenon is most commonly elicited by means of a pendulum that is made to swing in the subject's frontal plane. When a light-attenuating filter is put in front of one eye, the pendulum appears to swing in and out of the plane, following a more or less ellipsoidal trajectory. The Pulfrich phenomenon may also arise spontaneously, for example, in the presence of optic neuropathy, and may then cause multiple problems in daily life, especially when judging paths of moving objects. A truly monocular counterpart called the Lazy Shadow has been discovered recently. See the Pulfrich page for more information [B].
| Page 1 2 3 4 Next |