Vigabatrin is a gamma-aminobutyric acid (GABA) transaminase inhibitor that is effective in the treatment of partial epilepsy and infantile spasms. It is generally much appreciated by patients. Unfortunately, vigabatrin treatment is associated with a considerable risk of insidiously progressive and irreversible visual field defects. The question often arises about the best way to test for vigabatrin-associated damage (VAD) to the visual system. The pathophysiology of VAD is largely unknown and is possibly both multifactorial and multifocal. Hence, attempts to answer the question must draw on the collected, empirical experience with various tests. The following is by no means a complete review but an attempt to provide practically applicable answers to the best test question, based on personal experience with 80+ patients and a variety of tests [1-4]. The latter included light-reflex confrontation testing, manual kinetic ("Goldmann") perimetry, high-pass resolution perimetry, and rarebit perimetry, as described elsewhere (see Index). A search on PubMed will return around 200 references, including several reviews. In the interest of brevity, none will be cited here. I have no experience with electrophysiological evaluations.
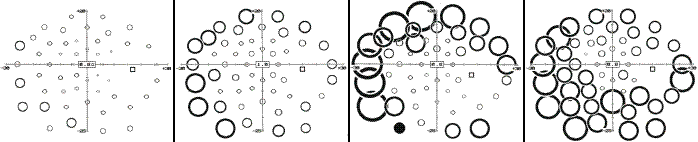
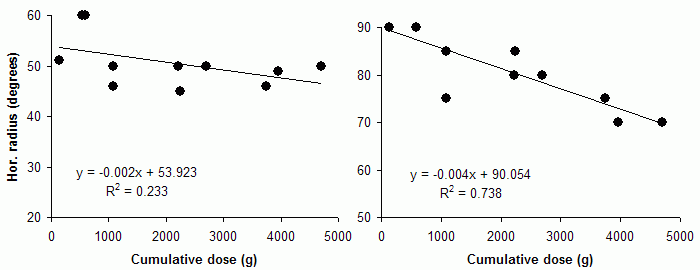
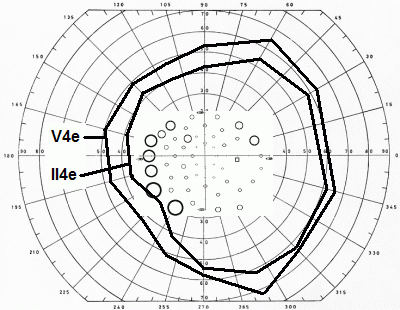
Most investigators have had experience with one type of test only, which makes comparisons of techniques difficult. The pioneer studies all employed Goldmann perimeters and documented peripheral field shrinkage, with little or no change in the central visual field, and without subjective corollaries. The combination of visual field contractions and lack of symptoms was initially commonly viewed as unspecific features of the underlying disorders, but an increasing number of reports attested to their validity and also a relationship with the cumulative vigabatrin dose (left: nasal field limit, right: temporal field limit, target: V4e; unpublished data):

 Nowadays, most clinics have replaced their Goldmann-type perimeters with computer-assisted devices. The exchange has undoubtedly led to better perimetry for the vast majority of patients.
However, in the case of VAD, the customary concentration on the central visual field appears unfortunate in the light of the pioneer studies. Meanwhile, additional evidence for primarily peripheral visual field damage has come from a perhaps unexpected direction, namely, the discovery of an unusual form of wasting of the retinal nerve fiber layer (NFL) in vigabatrin-treated subjects. The pattern of atrophy suggests that NFL bundles are wasted in order of length, much like the pattern of damage in peripheral neuropathies. While optic atrophy generally is not considered a major feature of VAD and actually has been denied by many investigators, these views are understandable in the light of the unusual distribution of NFL wasting and its inconspicuous nature. Nevertheless, goal-directed examinations have revealed not only a near 100% prevalence of atrophy among subjects with field defects, but also a possibility to grade its severity: the severity is correlated with the cumulative vigabatrin dose. Similar correlations apply to field limits.
Nowadays, most clinics have replaced their Goldmann-type perimeters with computer-assisted devices. The exchange has undoubtedly led to better perimetry for the vast majority of patients.
However, in the case of VAD, the customary concentration on the central visual field appears unfortunate in the light of the pioneer studies. Meanwhile, additional evidence for primarily peripheral visual field damage has come from a perhaps unexpected direction, namely, the discovery of an unusual form of wasting of the retinal nerve fiber layer (NFL) in vigabatrin-treated subjects. The pattern of atrophy suggests that NFL bundles are wasted in order of length, much like the pattern of damage in peripheral neuropathies. While optic atrophy generally is not considered a major feature of VAD and actually has been denied by many investigators, these views are understandable in the light of the unusual distribution of NFL wasting and its inconspicuous nature. Nevertheless, goal-directed examinations have revealed not only a near 100% prevalence of atrophy among subjects with field defects, but also a possibility to grade its severity: the severity is correlated with the cumulative vigabatrin dose. Similar correlations apply to field limits.
On the basis of the above considerations, it is submitted that the question of the best test should be subdivided into categories that take individual capacity and test availability into account. The following categories will be addressed:
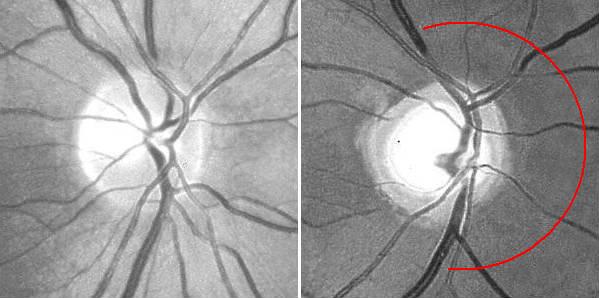
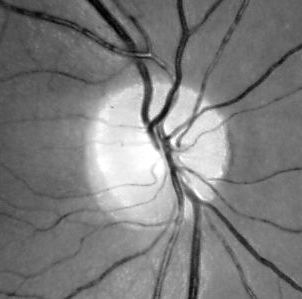
Additionally, fundus photography or NFL imaging is highly recommended in all instances, as these techniques provide objective records of the state of the NFL. The experience required for critical evaluation will come natural once a routine has been established. Particular attention should be payed to the state of the nasal disc border and the corresponding NFL sector, where wasting first appears. Secondary image processing is helpful to emphasize the NFL, e g, by means of the free NFLE software. The images shown below may help to highlight the differences between the NFLs of a normal subject (left) and a subject with advanced vigabatrin-associated atrophy (right). The latter shows a total loss of nasal and arcuate nerve fiber bundles, in the form of so-called C-shaped atrophy.
As to optimum intervals between repeat examinations, I have never seen convincing progression over time intervals shorter than one year. Hence, one-year intervals appear perfectly adequate.
It may be difficult to obtain meaningful visual field maps in some patients, not the least small children. In these situations, it is better to opt for fundus photography or NFL imaging than to struggle with possibly misleading field tests.
 However, to get an idea of the extent of the visual field, consider a reversed confrontation-type test. Two examiners are needed, one in front of the test subject and one behind. The former has the task of attracting fixation and monitoring
re-fixations. The latter examiner stands behind the subject and advances both hands, one on each side of the subject, from a position well behind the subject's ears, towards the subject's line of sight. A normal subject should easily detect the examiner's hands as they pass an imaginary transversal line through the outer canthi and should change his or her gaze direction to the one or the other hand. If the examiner has to advance his or her hands well beyond the inter-canthal line before a re-fixation occurs, temporal field contraction is very likely. Nasal contractions cannot be recognized in this type of test because of the lack of anatomical landmarks, so this is a test for temporal contractions only. It follows that there is little point in trying monocular testing.
However, to get an idea of the extent of the visual field, consider a reversed confrontation-type test. Two examiners are needed, one in front of the test subject and one behind. The former has the task of attracting fixation and monitoring
re-fixations. The latter examiner stands behind the subject and advances both hands, one on each side of the subject, from a position well behind the subject's ears, towards the subject's line of sight. A normal subject should easily detect the examiner's hands as they pass an imaginary transversal line through the outer canthi and should change his or her gaze direction to the one or the other hand. If the examiner has to advance his or her hands well beyond the inter-canthal line before a re-fixation occurs, temporal field contraction is very likely. Nasal contractions cannot be recognized in this type of test because of the lack of anatomical landmarks, so this is a test for temporal contractions only. It follows that there is little point in trying monocular testing.
As elaborated
elsewhere
on this site, the normal outer field limits are determined by facial contours. Hence, when swinging a light from behind the subject towards his or her line of sight, the subject should see the light at precisely the same moment as the examiner sees its reflection appear in the subject's cornea. This is a handy pass-fail test for contractions. A normal result reliably indicates the absence of field contractions whereas an abnormal result points to the need of formal testing. The sole disadvantage of this test is that the reflection's minuscule size poses considerable demands on the examiner's acuity. The reflection is quite hard to capture photographically:
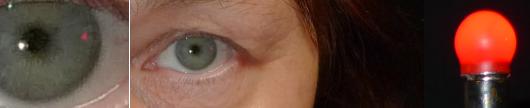
The extreme temporal periphery is likely to be first affected in early VAD as this part of the field is served by the very longest nerve fibers. Normally extending to some 110°, much of the temporal field extends outside the perimeter bowl.
However, it is a simple matter to arrange access by
tweaking the classical tangent screen technique.
All that is needed is a vacant office corner provided with suitable angle marks at eye height. A small mirror placed in the very
corner can serve both as a fixation mark and as an aid for monitoring fixation by the examiner,
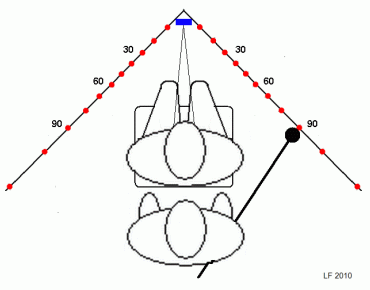 who should stand behind the subject.
Recalling that the goal is to map any field contractions, i e, blind areas, there is little point in fiddling with small test targets. Actually, a tennis ball that has been spray-painted in a contrasting color and stuck on to a simple wand should serve very well and will not leave scratch marks on the wall. The wand may double as a yardstick to position the subject correctly. Similarly, the ordinary room lights should serve well unless they throw distracting shadows in the test area. There is no need to go outside the horizontal meridian or to cover one eye unless mapping of nasal field limits is thought desirable.
who should stand behind the subject.
Recalling that the goal is to map any field contractions, i e, blind areas, there is little point in fiddling with small test targets. Actually, a tennis ball that has been spray-painted in a contrasting color and stuck on to a simple wand should serve very well and will not leave scratch marks on the wall. The wand may double as a yardstick to position the subject correctly. Similarly, the ordinary room lights should serve well unless they throw distracting shadows in the test area. There is no need to go outside the horizontal meridian or to cover one eye unless mapping of nasal field limits is thought desirable.
Like all other perimeters, the Goldmann perimeter cannot access the outermost part of the normal temporal visual field. Hence, truncation is inevitable in this area, indicating that the very earliest stages of VAD may be missed. The outermost part of the nasal field is accessible but VAD progresses at a slower rate in this region, as shown above. The difference is presumably attributable to differences in the lengths of the NFL bundles serving the temporal and nasal borders: the former normally span some 110° - 15° and the latter some 60° + 15°, a 20° difference. Upper and lower field limits are normally and variably obscured by the upper and lower eyelids and mapping of the upper and lower bounds cannot be expected to return meaningful results in the VAD setting.
Recognizing the above-mentioned limitations, I would argue that the optimum Goldmann procedure involves concentration on the temporal and nasal extremes of the horizontal meridian. Personally, I start with the II4e target, with 5 repeats at each end. If this turns out normal, there is no point in continuing. However, if the results are abnormal, I add the same number of tests using the V4e target, to assess the slope of the threshold surface. If the slope is steep, as expected with VAD, I am done. However, if the slope is shallow, other causes need to be considered and a full field examination is indicated.
The large variety of computer-assisted perimeters precludes a detailed listing of the best testing routines. Generally speaking, any routine that targets the temporal and nasal limits should return results similar to the Goldmann perimeter, and with the same limitations, whereas central-field routines are likely to return abnormal results first at later stages of damage. Noting that the characteristic feature of VAD is contraction, there is little point in using threshold modes: supra-threshold testing should be both adequate and time-saving. Short test times may be particularly important in subjects in need of vigabatrin therapy. Routines traceable to the so-called Esterman Grid [5] may prove useful in these regards.
The montage below shows the results of Goldmann and high-pass resolution perimetry in a subject with vigabatrin-associated optic atrophy. There is a quite pronounced contraction, particularly temporally, yet the central field shows a minimal nasal depression only. The latter is more pronounced below, fitting with the more marked wasting of the upper part of the NFL.
![]()
The following panel exemplifies high-pass resolution perimetry results from a normal subject (left) and three subjects with advanced VAD. Note sparing of the central-most field.